alvin l nunley
Site Supporter
Question; does alcohol attract water?
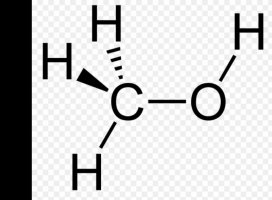
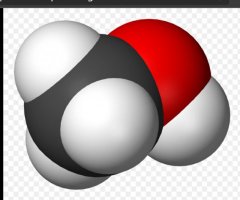
Looking at the 2 pictures, you can see that there is an oxygen atom attached to one Hydrogen atom. I've been told by a chemist that the Oxygen atom wants to be bonded to 2 hydrogen atoms. There is a very strong attraction between the 2 atoms. If that oxygen atom is exposed to the air, and a hydrogen atom finds it and bonds do it, it becomes water H2O.
My chemist friend says; alcohol does not attract water, it attracts hydrogen atoms and becomes water.
The hydrogen atom, being the smallest atom on the periodic table, can get into places the water molecule could never get into.
Looking at the 2 pictures, you can see that there is an oxygen atom attached to one Hydrogen atom. I've been told by a chemist that the Oxygen atom wants to be bonded to 2 hydrogen atoms. There is a very strong attraction between the 2 atoms. If that oxygen atom is exposed to the air, and a hydrogen atom finds it and bonds do it, it becomes water H2O.
My chemist friend says; alcohol does not attract water, it attracts hydrogen atoms and becomes water.
The hydrogen atom, being the smallest atom on the periodic table, can get into places the water molecule could never get into.